Your Location:Home > Products > Featured functional additives > UV polymerization inhibitor >2226-96-2
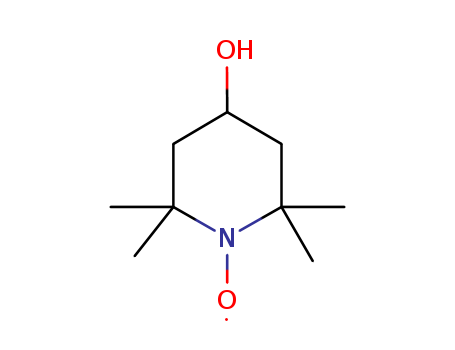

Product Details
|
Synthesis Reference(s) |
Synthetic Communications, 19, p. 3509, 1989 DOI: 10.1080/00397918908052760 |
|
Flammability and Explosibility |
Nonflammable |
|
Biological Activity |
Superoxide scavenger that displays neuroprotective, anti-inflammatory and analgesic effects. |
|
General Description |
4-Hydroxy-TEMPO is a 4-substituted 2,2,6,6-tetramethylpiperidyl-1-oxy (TEMPO) derivative. It is a low-molecular weight compound and has been proposed as superoxide dismutase mimic. |
InChI:InChI=1/C9H18NO2/c1-8(2)5-7(11)6-9(3,4)10(8)12/h7,11H,5-6H2,1-4H3
The reactions of sterically hindered ami...
The composite EPR spectra of a spin prob...
The reactive nitroxides 2,2,6,6-tetramet...
The use of UV/visible light irradiation ...
Chemotherapy is a general treatment opti...
-
Abstract: HSV disease is distributed wor...
The single electron transfer-nitroxide r...
The reactions of 4-hydroxy-2,2,6,6-tetra...
The method comprises the following steps...
We report the aggregation-induced photos...
In this study, 3-hydroxymethyl-2,2,5,5-t...
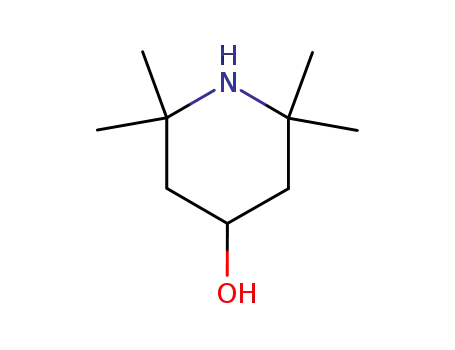
4-hydroxy-2,2,6,6-tetramethylpiperidine

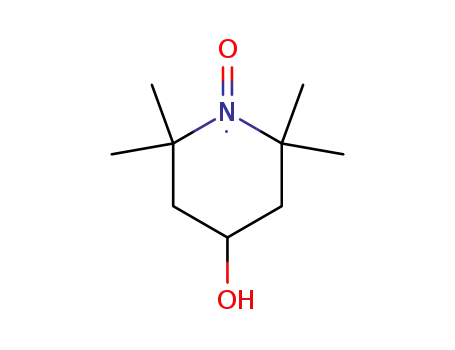
TEMPOL

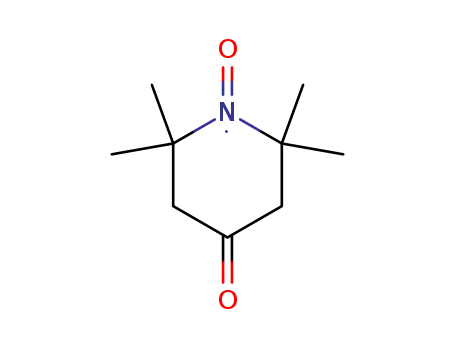
4-oxo-2,2,6,6-tetramethylpiperidin-oxyl
| Conditions | Yield |
|---|---|
|
With
potassium superoxide;
In
water; acetonitrile;
at 21.84 ℃;
Inert atmosphere;
Irradiation;
|
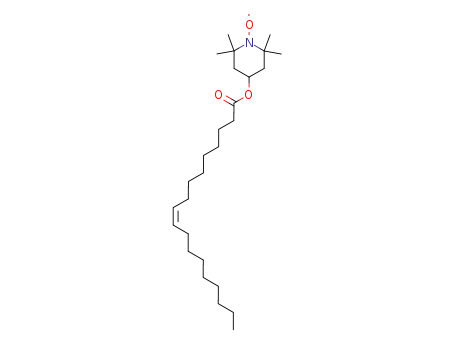
TEMPO-OL


TEMPOL

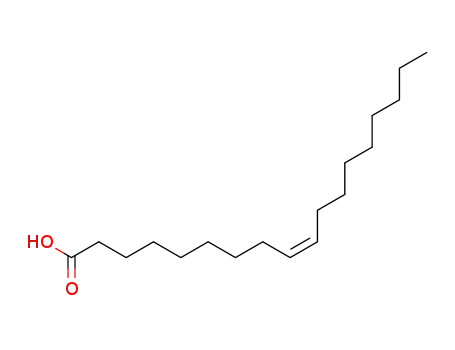
cis-Octadecenoic acid
| Conditions | Yield |
|---|---|
|
With
water; lipase;
for 37h;
Product distribution;
|

4-hydroxy-2,2,6,6-tetramethylpiperidine
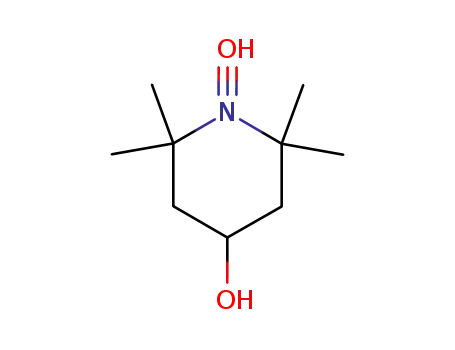
tempol
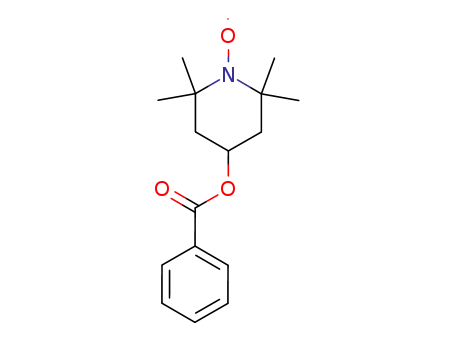
4-(benzyloxycarbonyl)-2,2,6,6-tetramethylpiperidine-1-oxyl

TEMPO-OL

tempol
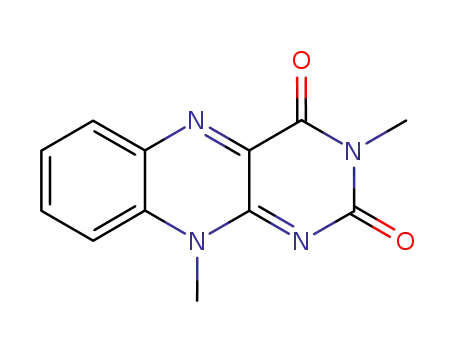
3,10-dimethylisoalloxazine
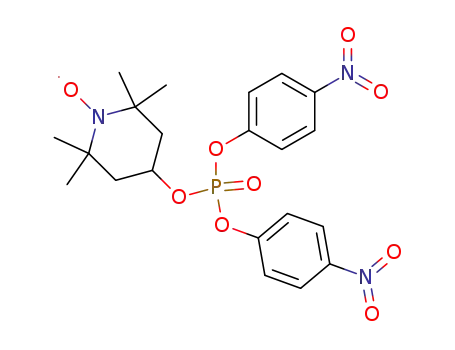
1-oxy-2,2,6,6-tetramethyl-4-piperidinyl phosphate
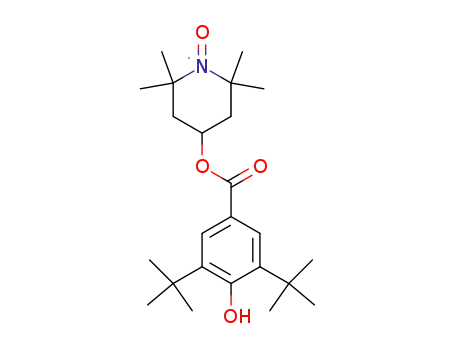
C24H38NO4
CAS:74150-27-9
CAS:1837-55-4
CAS:52550-44-4