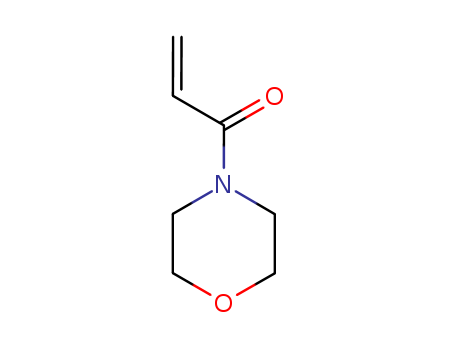

Product Details
|
Flammability and Explosibility |
Nonflammable |
|
Synthesis |
A solution of 0.04 mol of the corresponding amine in 20 ml of anhydrous methylene chloride was slowly added at 0-5°C to 0.02 mol of acryloyl chloride in 20 ml of anhydrous methylene chloride. The mixture was stirred for 3 h at room temperature in an inert atmosphere, and the precipitate was filtered off and washed with methylene chloride (2 × 10 ml). The organic layer was washed in succession with 5 ml of water and 5 ml of a saturated solution of NaHCO3 and dried over Na2SO4, the solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel using hexane-ethyl acetate (5 : 1 to 1 : 1) as eluent. 4-Acryloylmorpholine, Yield 1.78 g (63%). IR spectrum, ν, cm-1: 2857, 1647, 1612, 1439, 1263, 1238, 1115, 1038, 953. 1H NMR spectrum, δ, ppm: 3.51-3.73 m (8H, NCH2CH2O), 5.72 d.d (1H, 3-Hcis, 3J = 10.6, 2J = 1.9 Hz), 6.29 d.d (1H, 3-Htrans, 3J = 16.7, 2J = 1.9 Hz), 6.57 d.d (1H, 2-H, J = 16.7, 10.6 Hz). 13C NMR spectrum, δC, ppm: 41.74 and 45.66 (CH2N), 66.22 (CH2O), 126.64 (C2), 127.69 (C3), 164.92 (C1). Mass spectrum, m/z (Irel, %): 141 (36) [M]+, 140 (12), 126 (58), 112 (22), 111 (15), 110 (15), 109 (12), 98 (10), 96 (26), 86 (72), 83 (13), 70 (14), 68 (14), 57 (17), 56 (86), 55 (100), 42 (23).Fig. The synthetic method 2 of 4-Acryloylmorpholine |
InChI:InChI=1/C10H12N2O2/c1-7-4-3-5-9(6-7)10(14)12-11-8(2)13/h3-6H,1-2H3,(H,11,13)(H,12,14)
We report herein the first KHMDS-catalyz...
The invention provides a catalytic synth...
Transition-metal-catalyzed asymmetric hy...
The invention discloses a synthesis meth...
formic acid

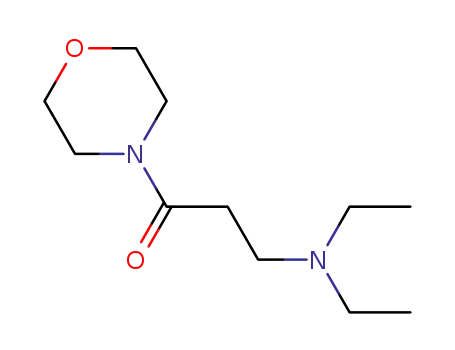
3-(diethylamino)-1-morpholinopropan-1-one

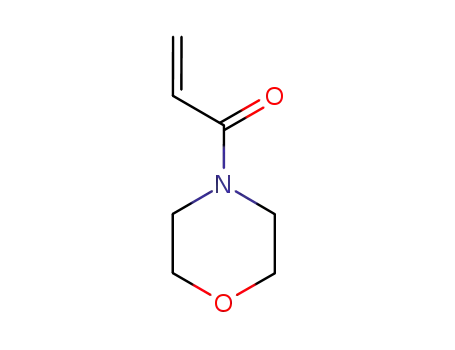
N-Acryloylmorpholine

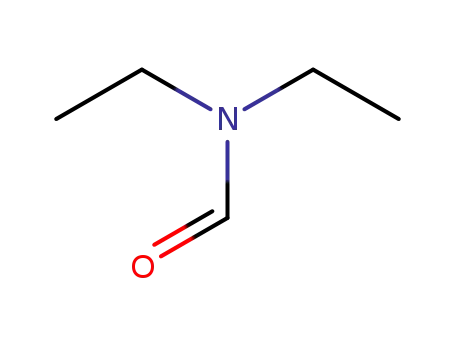
N-formyldiethylamine
| Conditions | Yield |
|---|---|
|
With
10H-phenothiazine;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 140 ℃;
for 5.75h;
Inert atmosphere;
|
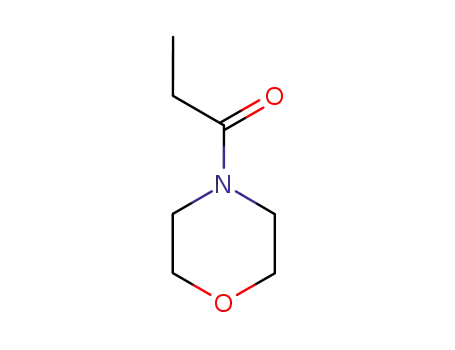
1-morpholinopropane-1-one


N-Acryloylmorpholine
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate; oxygen; palladium diacetate;
In
dimethyl sulfoxide;
at 90 - 120 ℃;
for 36h;
under 760.051 Torr;
Reagent/catalyst;
Temperature;
|
90% |
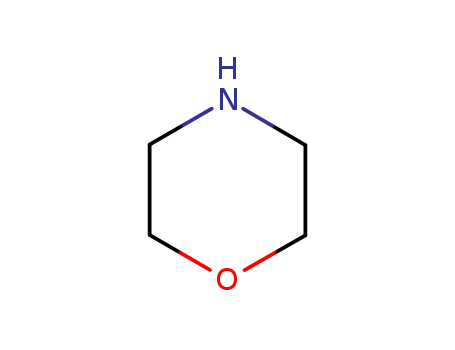
morpholine
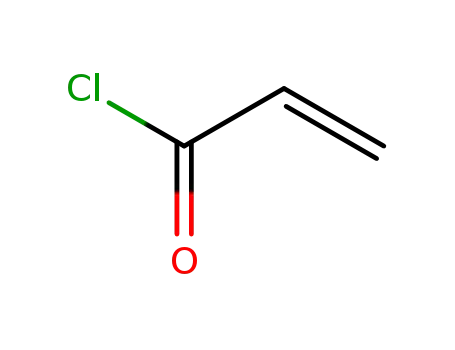
acryloyl chloride
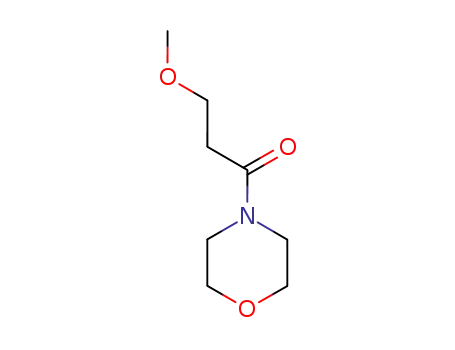
4-(3-methoxy-propionyl)-morpholine
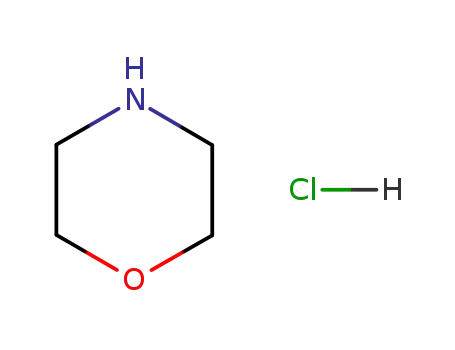
morpholin hydrochloride
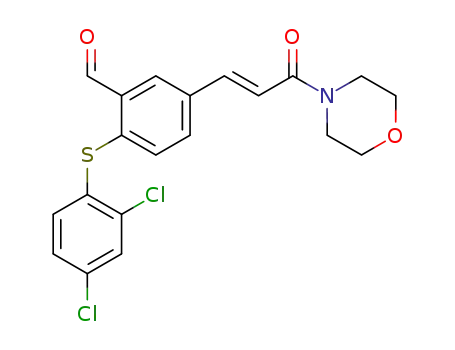
(2,4-dichlorophenyl)[2-formyl-4-(E-((1-morpholinyl)carbonyl)ethenyl)phenyl]sulfide
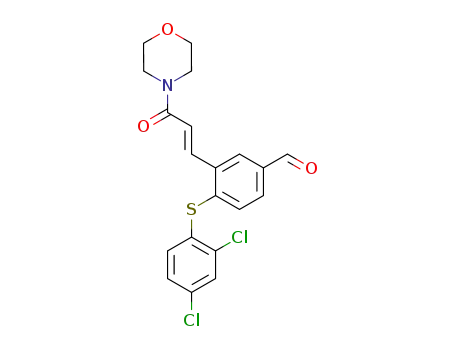
2,4-dichlorophenyl 2-((E)-((1-morpholino)carbonyl)ethenyl)-5-formylphenyl sulfide
N-(2-bromophenyl)-N-methylacetamide
CAS:74150-27-9
CAS:2097600-97-8
CAS:90-93-7