Your Location:Home > Products > Photoinitiator > High-end ink photoinitiator >478556-66-0
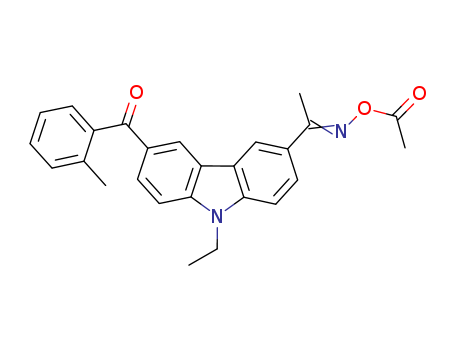

Product Details
|
General Description |
1-[9-Ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl]ethanone 1-(O-acetyloxime) is a chemical compound that belongs to the carbazole family. It is a ketone with an oxime functional group attached to it. 1-[9-Ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl]ethanone 1-(O-acetyloxime) has a complex structure consisting of a carbazole ring with an ethyl and 2-methylbenzoyl substituent. The presence of the oxime functional group signifies that it can potentially act as a chelating agent, forming coordination complexes with metal ions. 1-[9-Ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl]ethanone 1-(O-acetyloxime) may have potential applications in organic synthesis, coordination chemistry, and possibly pharmaceutical research. Its precise properties and uses would need to be determined through further experimental study. |
InChI:InChI=1/C26H24N2O3/c1-5-28-24-12-10-19(17(3)27-31-18(4)29)14-22(24)23-15-20(11-13-25(23)28)26(30)21-9-7-6-8-16(21)2/h6-15H,5H2,1-4H3
The invention discloses a green synthesi...
The present invention provides a polymer...
1-{6-(2-Methylbenzoyl)-N-ethylcarbazole-...
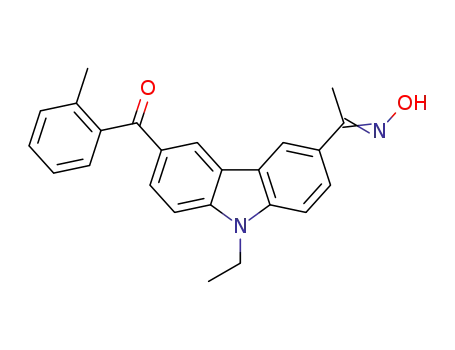
(6-o-methylbenzoyl-N-ethylcarbazol-3-yl)ethanone oxime

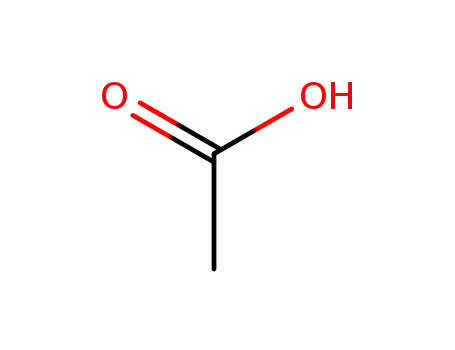
acetic acid

![ethanone-1-[9-ethyl-6-(2-methylbenzoyl)-9H-carbazole-3-yl]-1-(O-acetyloxime)](/upload/2024/10/0903f40e-ea8e-4af5-bb02-0704775b3001.png)
ethanone-1-[9-ethyl-6-(2-methylbenzoyl)-9H-carbazole-3-yl]-1-(O-acetyloxime)
| Conditions | Yield |
|---|---|
|
With
boric acid;
In
water; chlorobenzene;
Solvent;
Reflux;
|
80% |
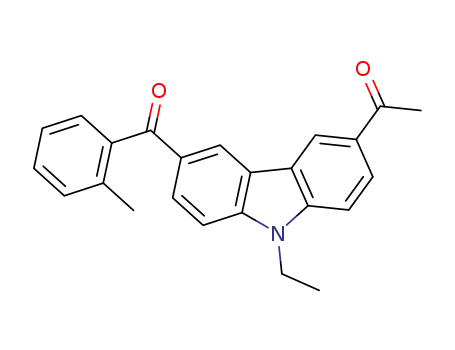
3-acetyl-6-(o-methyl benzoyl)-N-ethylcarbazole

sodium acetate

![ethanone-1-[9-ethyl-6-(2-methylbenzoyl)-9H-carbazole-3-yl]-1-(O-acetyloxime)](/upload/2024/10/0903f40e-ea8e-4af5-bb02-0704775b3001.png)
ethanone-1-[9-ethyl-6-(2-methylbenzoyl)-9H-carbazole-3-yl]-1-(O-acetyloxime)
| Conditions | Yield |
|---|---|
|
3-acetyl-6-(o-methyl benzoyl)-N-ethylcarbazole; sodium acetate;
With
hydroxylamine hydrochloride; tetrabutylammomium bromide;
In
water; 1,2-dichloro-ethane;
at 70 ℃;
for 7h;
With
toluene-4-sulfonic acid;
In
water; 1,2-dichloro-ethane;
for 6h;
Reflux;
|
89.8% |

3-acetyl-6-(o-methyl benzoyl)-N-ethylcarbazole

sodium acetate

acetic acid
N-ethylcarbazole
CAS:74150-27-9
CAS:253585-83-0