Your Location:Home > Products > Photoinitiator > Other photoinitiator >15206-55-0

Product Details
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Purify the ester by radial chromatography (diethyl ether/hexane, 1:1), and dry it at 110-112o/6mm. [Meyers & Oppenlaender J Am Chem Soc 108 1989 1986, Beilstein 10 IV 2738.] |
|
Definition |
ChEBI: The methyl ester of phenylglyoxylic acid with methanol. Metabolite observed in cancer metabolism. |
InChI:InChI=1/C9H8O3/c1-12-9(11)8(10)7-5-3-2-4-6-7/h2-6H,1H3
Herein, we report on rhodium catalysed c...
Photosensitized oxidation of tellurides ...
-
-
Sodium bromide is an effective catalyst ...
The Fe(II)-cysteine-containing peptide c...
An efficient access to vicinal dioxoalka...
-
A rapid and efficient synthesis of α-ket...
A simple and efficient method is describ...
α-Keto acid esters can be easily prepare...
Methyl phenylglyoxylate, aniline and aro...
-
A novel one-pot synthesis of aryl α-keto...
Methyl mandelate undergoes quantitative ...
We report a direct synthesis of 2-thioxo...
-
Oxidation is an important class of react...
The reactions of 4,5-epoxy-2-decenal wit...
The enantiospecific total synthesis of t...
Evidence from product studies and radica...
Co(salen) catalysed oxygenation of 4-sub...
Oxidation of benzylic and allylic substr...
Direct synthesis of arylglyoxylic esters...
The combination of iodine and iodine pen...
The mechanism of rhodium-COD-catalyzed h...
Selective oxidation of α-hydroxy esters ...
Heterogeneous aerobic oxidative esterifi...
Two pathways for N-hydroxyphthalimide (N...
Electrochemical oxygen reduction reactio...
An efficient copper-catalyzed tandem oxi...
-
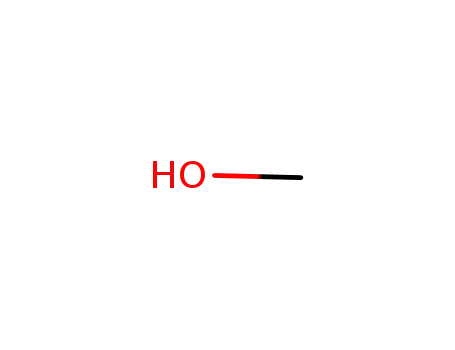
methanol

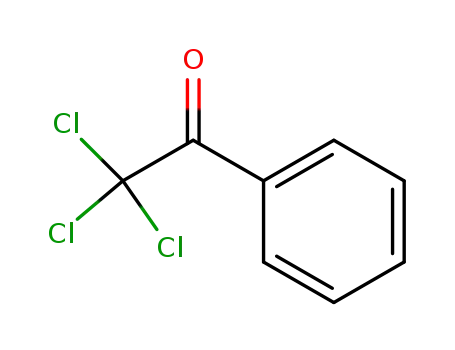
2,2,2-trichloroacetophenone

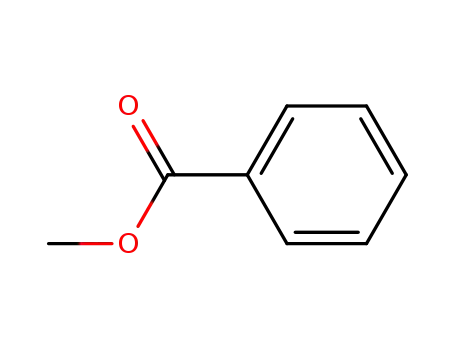
benzoic acid methyl ester

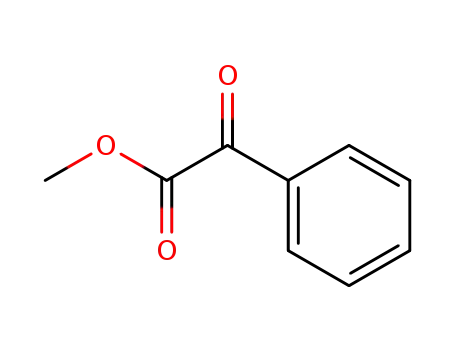
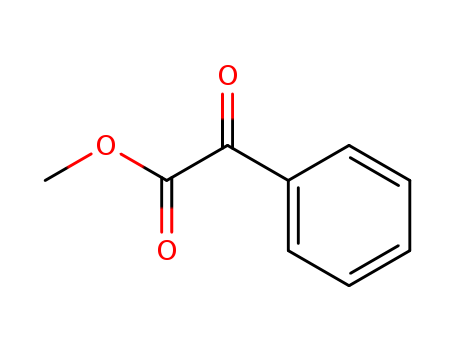
methyl 2-oxo-2-phenylacetate

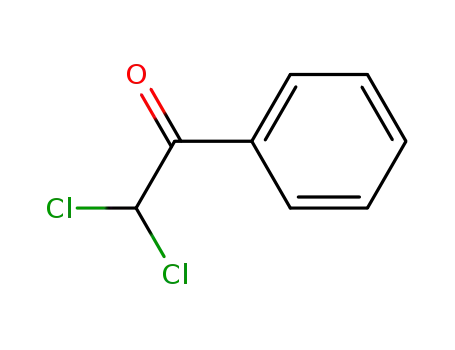
2,2-dichloroacetophenone
| Conditions | Yield |
|---|---|
|
for 16.5h;
Irradiation;
|
6.4% 6% 22.7% |
|
at 25 ℃;
for 1h;
Product distribution;
Mechanism;
Irradiation;
Effects of oxygen, ring substituents, quenching, (piperylene) and sensitization, (benzophenone).;
|
10.6 % Chromat. 9.7 % Chromat. 12.3 % Chromat. |

2,2,2-trichloroacetophenone


benzoic acid methyl ester


methyl 2-oxo-2-phenylacetate


2,2-dichloroacetophenone
| Conditions | Yield |
|---|---|
|
In
methanol;
for 16.5h;
Irradiation;
|
22.7% 6.4% 6% |
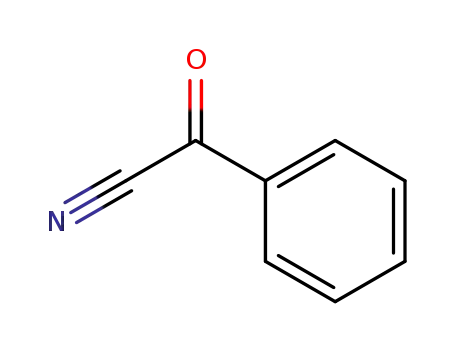
benzoyl cyanide

methanol
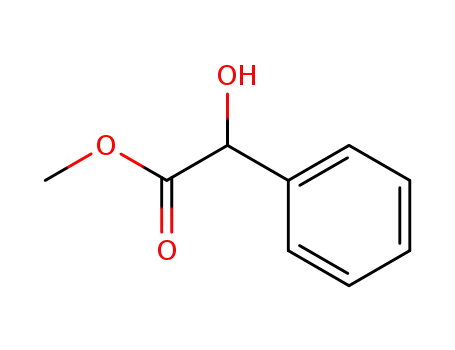
(RS)-methyl mandelate
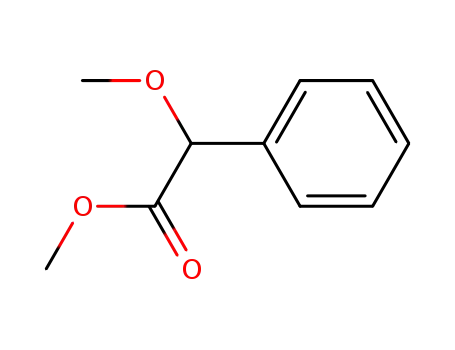
methyl 2-methoxy-2-phenylacetate
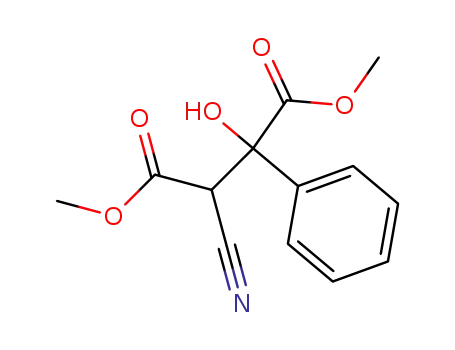
3-cyano-2-hydroxy-2-phenyl-succinic acid dimethyl ester
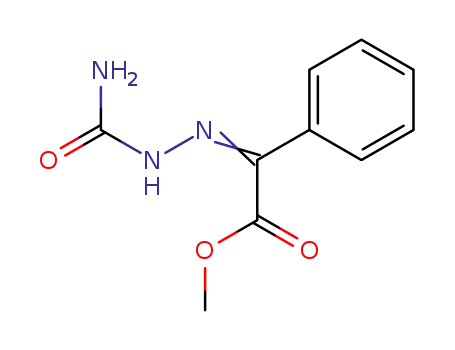
methyl benzoylformate semicarbazone
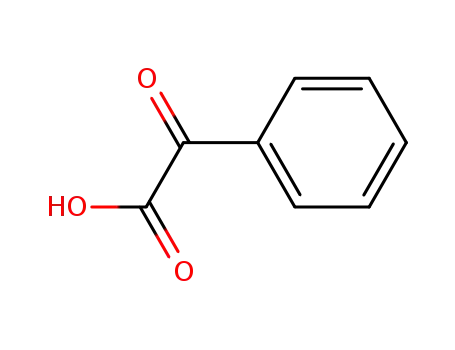
Benzoylformic acid
methyl benzoyl formate phenylhydrazone
CAS:86428-83-3
CAS:606-28-0