Your Location:Home > Products > Photoinitiator > Other photoinitiator >606-28-0
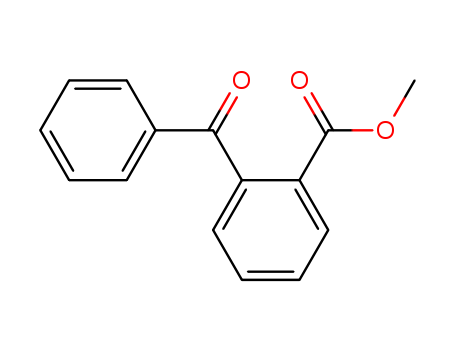

Product Details
|
Preparation |
Methyl-2-benzoylbenzoate can be synthesized from the reaction between corresponding 2-substituted benzoic acid and thionyl chloride in methanol. |
|
Flammability and Explosibility |
Nonflammable |
|
General Description |
Methyl-2-benzoylbenzoate is a 2-acylarylcarboxylate. It can undergo asymmetric transfer hydrogenation reaction in propanol in the presence of a Ruthenium catalyst. Methyl-2-benzoylbenzoate is formed as one of the reaction products during the reaction between methyl benzoate and lithium 2,2,6,6-tetramethylpiperidide (LiTMP) at -117°C. |
InChI:InChI=1/C15H12O3/c1-18-15(17)13-10-6-5-9-12(13)14(16)11-7-3-2-4-8-11/h2-10H,1H3
A method for preparing an alkyl 2-benzoy...
Herein we report ketones as feedstock ma...
Acyl radicals have been generated from α...
The presence of salicylic acid (10 mol-%...
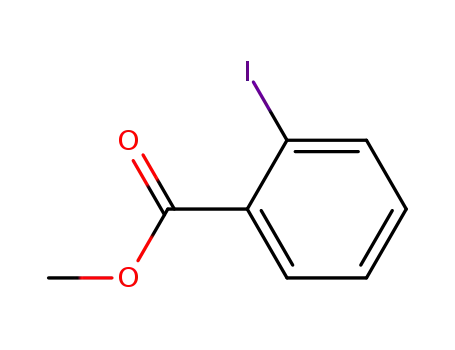
o-iodo-methyl-benzoic acid

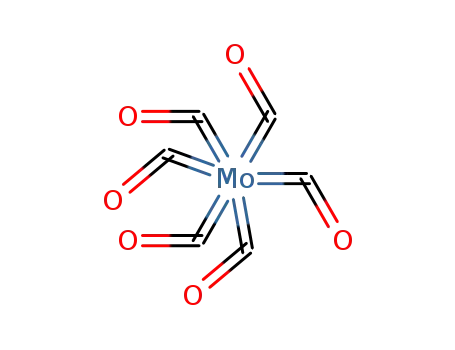
molybdenum hexacarbonyl

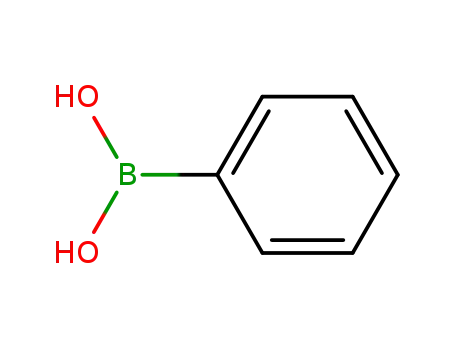
phenylboronic acid

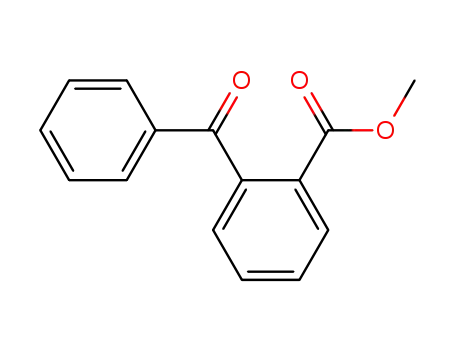
methyl o-benzoylbenzoate
| Conditions | Yield |
|---|---|
|
With
C35H20F34NO3(1-)*Pd(2+)*Cl(1-); N-ethyl-N,N-diisopropylamine;
In
water;
at 140 ℃;
for 0.2h;
Reagent/catalyst;
Concentration;
Temperature;
Catalytic behavior;
Microwave irradiation;
|
73% |
|
With
palladium diacetate; potassium carbonate;
In
methoxybenzene;
at 140 ℃;
for 12h;
|
60% |
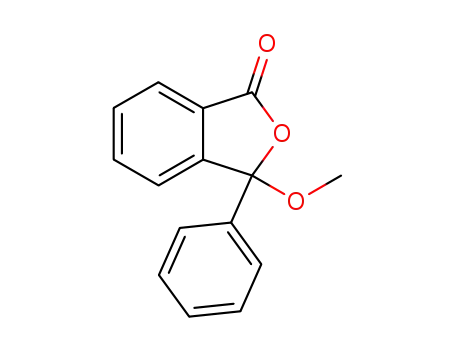
3-methoxy-3-phenyl-phthalide


methyl o-benzoylbenzoate
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid;
In
toluene;
for 6h;
Reflux;
|
99.2% |

diazomethane
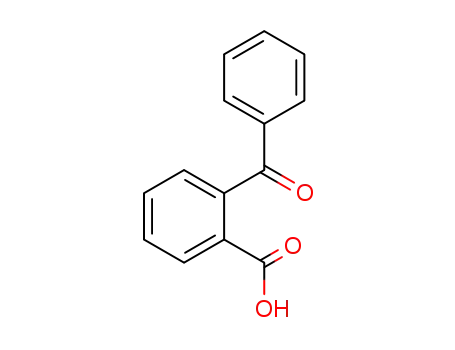
2-Benzoylbenzoic acid
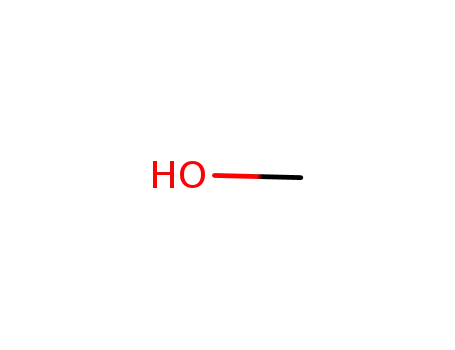
methanol
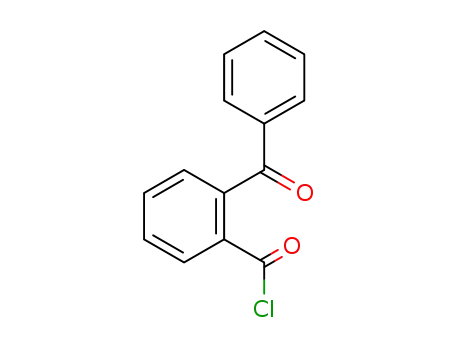
2-benzoylbenzoyl chloride
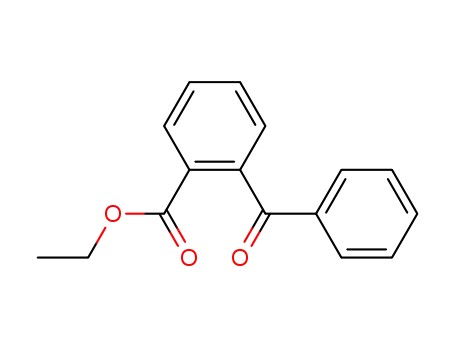
ethyl 2-benzoylbenzoate
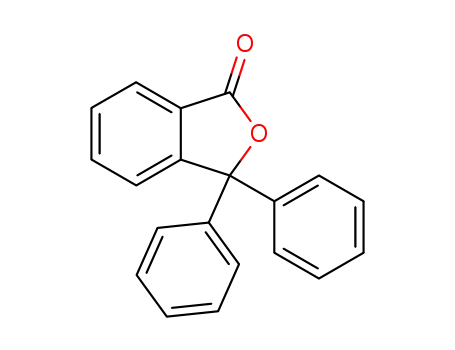
3,3-diphenyl-2-benzofuran-1(3H)-one
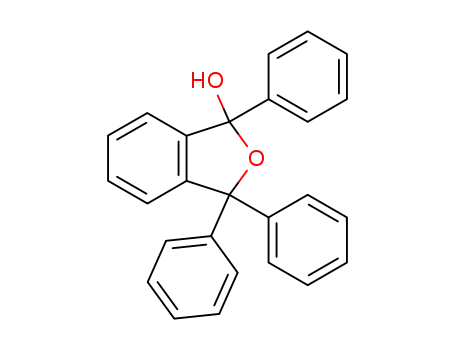
1-hydroxy-1,3,3-triphenylphthalan
o-dibenzoylbenzene
CAS:86428-83-3
CAS:15206-55-0
CAS:119313-12-1