Your Location:Home > Products > Pharma & Intermediates > Other intermediate >882-33-7
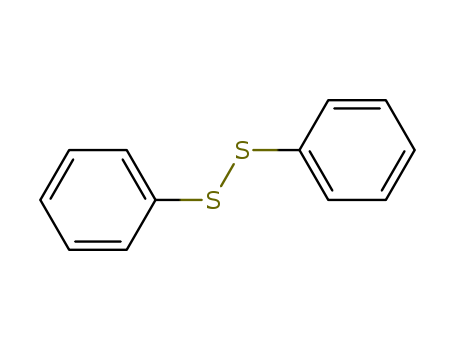

Product Details
|
Chemical Description |
Diphenyl disulfide is a compound with two phenyl groups attached to a sulfur atom. |
|
Reactions and Uses |
With Alkenes Ph2S2 adds cleanly to many dienes and alkenes upon catalysis by BF3.OMe2. The trifluoroacetoxysulfenylation of unsaturated esters, nitriles, amides, and carboxylic acids is possible with Ph2S2. As a Source of Thiiyl Radicals In the presence of radical initiators, upon thermolysis or photolysis, thiiyl radicals can be generated from PhSSPh. Therefore, the radicals generated add reversibly to alkynes and alkenes generating vinyl and alkyl radicals respectively (1). Reduction Ph2S2 undergoes reduction, which is a characteristic of disulfides. Hydride reagents such as super hydride and sodium borohydride can be used as reductants. Ph2S2 + 2 M → 2 MSPh (M = Li, Na, K) |
|
Preparation |
By heating and passing a stream of air over an ammoniacal solution of thiophenol. |
|
Synthesis Reference(s) |
Chemical and Pharmaceutical Bulletin, 35, p. 1770, 1987 DOI: 10.1248/cpb.35.1770Tetrahedron Letters, 25, p. 703, 1984 DOI: 10.1016/S0040-4039(01)80004-6 |
|
Purification Methods |
Crystallise the disulfide from MeOH. [Alberti et al. J Am Chem Soc 108 3024 1986]. Also crystallise it repeatedly from hot Et2O, then dry it in a vacuum at 30o over P2O5, fuse it under N2 and re-dry it; the whole procedure being repeated, with a final drying under a vacuum for 24hours. Alternatively, recrystallise it from hexane/EtOH solution. [Burkey & Griller J Am Chem Soc 107 246 1985, Beilstein 6 H 323, 6 IV 1560.] |
InChI:InChI=1/C12H10S2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H
Highly stabilized isoquinolinium methyli...
The kinetics of coupling of carbon radic...
Antitumor, antimicrobial, and phytotoxic...
In our research of precursors of tin rad...
-
-
We describe herein a green and efficient...
-
Here we report a visible-light-mediated ...
We report the first copper-catalyzed ole...
The synthesis of 2-sulfanyl-1,2-dihydro-...
Routes to mixed-chalcogen diplatinum com...
An electrocatalytic reduction of [(3-{[t...
The reactions of phenyl thiyl radicals w...
-
-
Adsorption of diphenyl disulfide (DPDS) ...
-
Tetrasulphur tetranitride and phenyl vin...
-
This is the first report on the synthesi...
Among external stimuli used to promote a...
An efficient, transition-metal free and ...
Organo disulfides represent an abundant ...
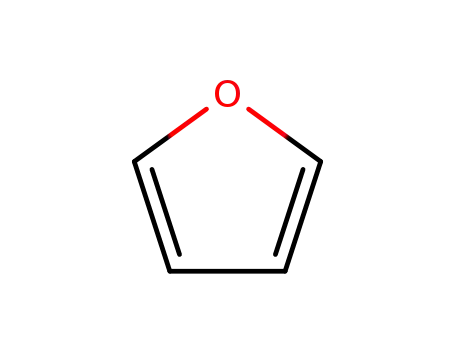
furan

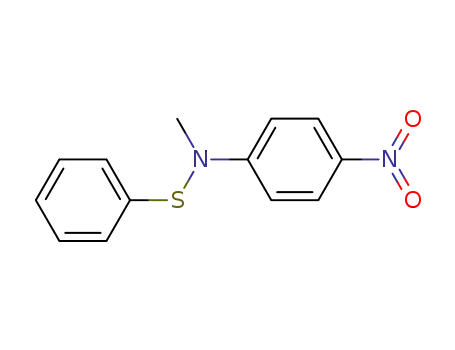
N-methyl-4-nitro-N-(phenylthio)benzenamine

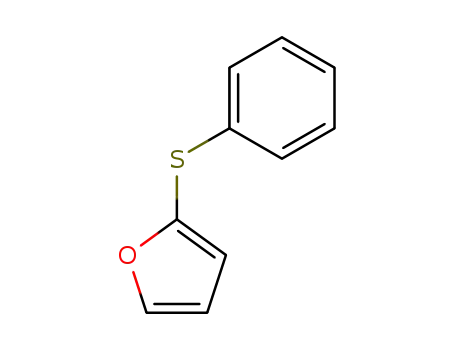
2-phenylthiofuran

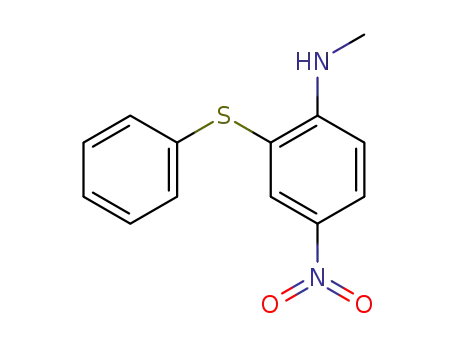
N-methyl-2-(phenylthio)-4-nitroaniline

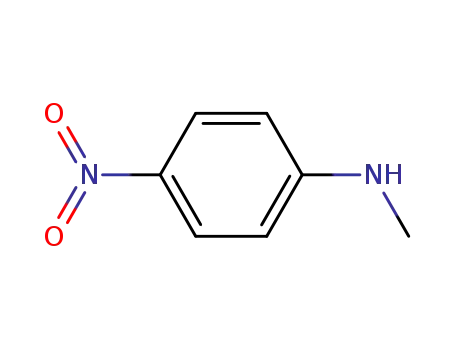
N-methyl(p-nitroaniline)

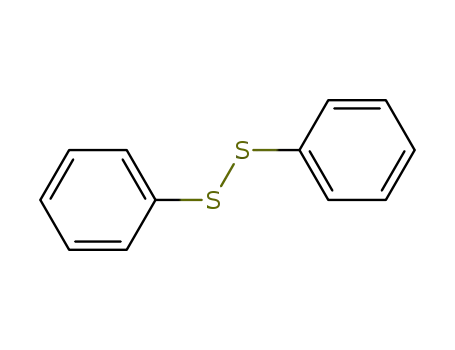
diphenyldisulfane
| Conditions | Yield |
|---|---|
|
at 85 ℃;
for 144h;
|
79% 25% 4% 40% |
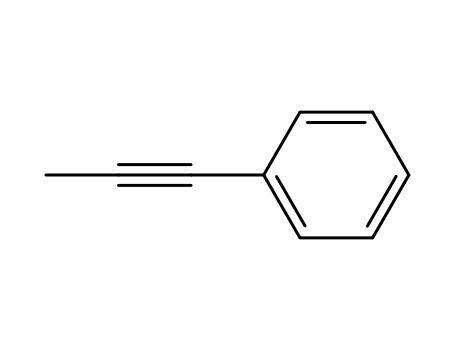
1-Phenylprop-1-yne

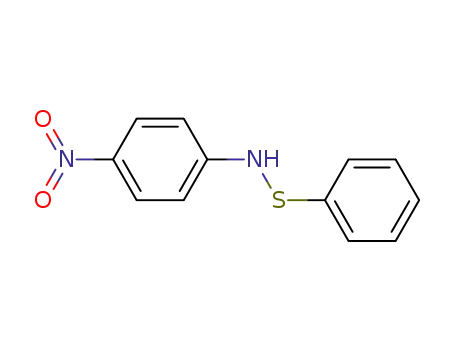
4'-nitrobenzenesulfenanilide

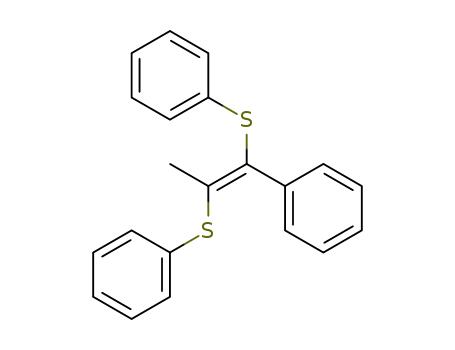
(E)-1-phenyl-1,2-bis(phenylthio)propene

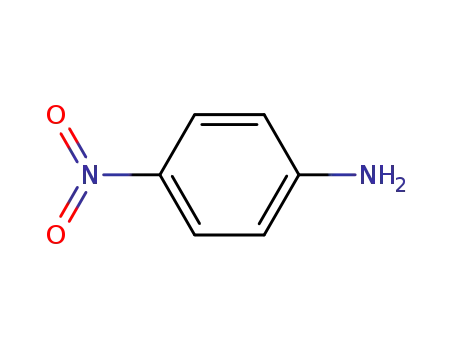
4-nitro-aniline


diphenyldisulfane

(E)-N-(4-nitrophenyl)-S-phenyl-S-<2-(phenylthio)-1-phenylpropen-1-yl>sulphimide
| Conditions | Yield |
|---|---|
|
With
boron trifluoride diethyl etherate;
In
dichloromethane;
for 0.75h;
Yield given. Further byproducts given. Yields of byproduct given;
Ambient temperature;
|
|
|
With
boron trifluoride diethyl etherate;
In
benzene;
for 0.75h;
Yield given. Further byproducts given. Yields of byproduct given;
Ambient temperature;
|
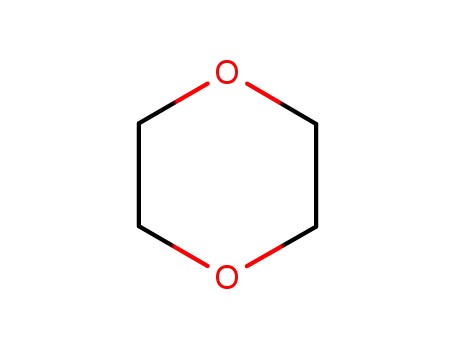
1,4-dioxane
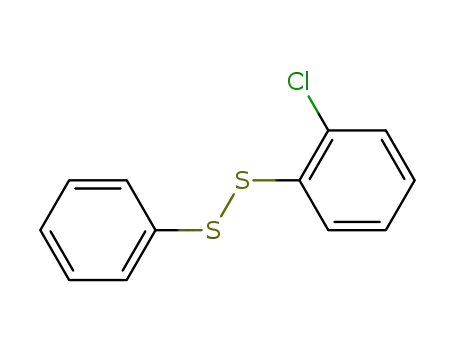
(2-chloro-phenyl)-phenyl disulfide
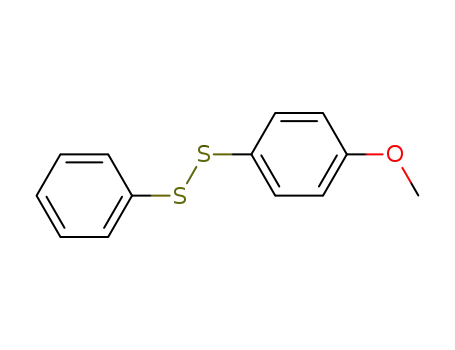
1-(4-methoxyphenyl)-2-phenyldisulfane
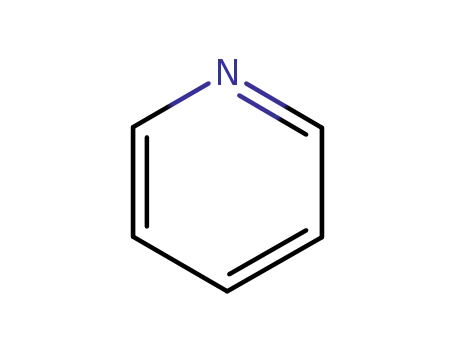
pyridine
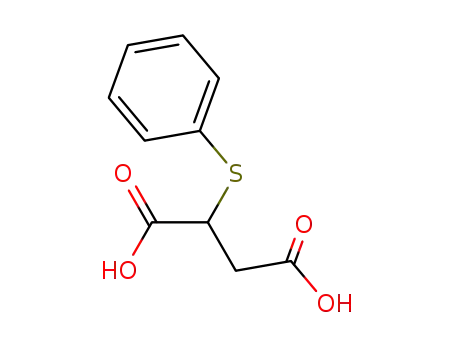
phenylsulfanyl-succinic acid
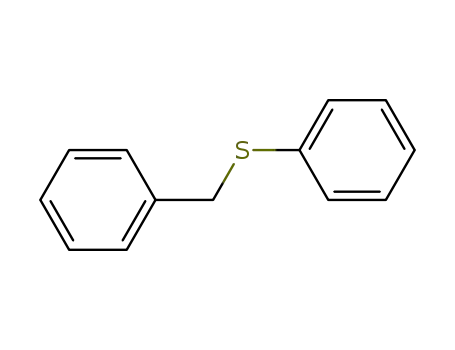
Benzyl phenyl sulfide
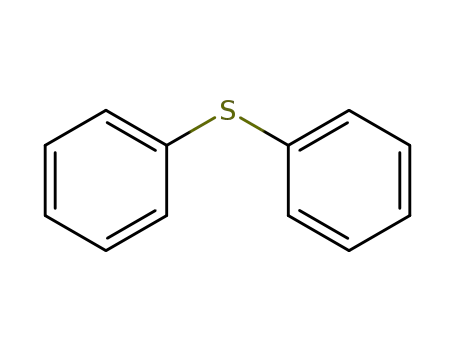
diphenyl sulfide
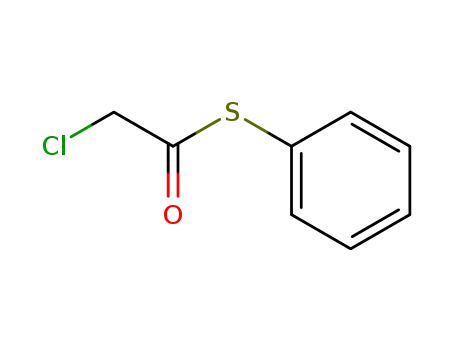
S-phenyl α-chlorothioacetate
CAS:66104-23-2
CAS:148757-88-4
CAS:86273-46-3
CAS:83310-97-8