Your Location:Home > Products > Photoinitiator > Other photoinitiator >83846-85-9

Product Details
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C20H16OS/c1-15-7-11-18(12-8-15)22-19-13-9-17(10-14-19)20(21)16-5-3-2-4-6-16/h2-14H,1H3
Nickel-catalyzed decarbonylative thioeth...
We have proven that pyridine-boryl compl...
Herein, we have reported a nickel-cataly...
Transition-metal-catalyzed coupling reac...
(4-bromophenyl)(phenyl)methanone

para-thiocresol

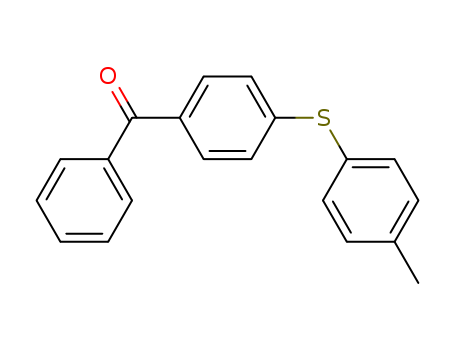
4-[(4-methylphenyl)sulfanyl]benzophenone
| Conditions | Yield |
|---|---|
|
With caesium carbonate; In dimethyl sulfoxide; at 25 ℃; for 1h; Irradiation; Inert atmosphere;
|
90% |
|
With caesium carbonate; In dimethyl sulfoxide; at 25 ℃; for 1h; Inert atmosphere; UV-irradiation;
|
90% |
|
With nickel(II) bromide dimethoxyethane; 4,4'-di-tert-butyl-2,2'-bipyridine; lithium bromide; In N,N-dimethyl-formamide; at 20 ℃; for 6h; Electrochemical reaction;
|
80% |
4-benzoylbenzoyl fluoride

para-thiocresol

4-[(4-methylphenyl)sulfanyl]benzophenone
| Conditions | Yield |
|---|---|
|
With (1,2-dimethoxyethane)dichloronickel(II); 1,3-bis-(diphenylphosphino)propane; sodium carbonate; In toluene; at 140 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
80% |
4-chlorobenzophenone
para-thiocresol
S-(4-methylphenyl) thiobenzoate
[4-(4-Chloro-phenoxy)-phenyl]-phenyl-methanone
2-(4-methylphenyl)-1,3-benzoxazole
(4-(benzoxazol-2-yl)phenyl)(phenyl)methanone
p-<(p-methylphenyl)sulfonyl>benzophenone
CAS:66104-23-2
CAS:71868-10-5
CAS:1107606-68-7
CAS:134-84-9